sodium-bicarbonate
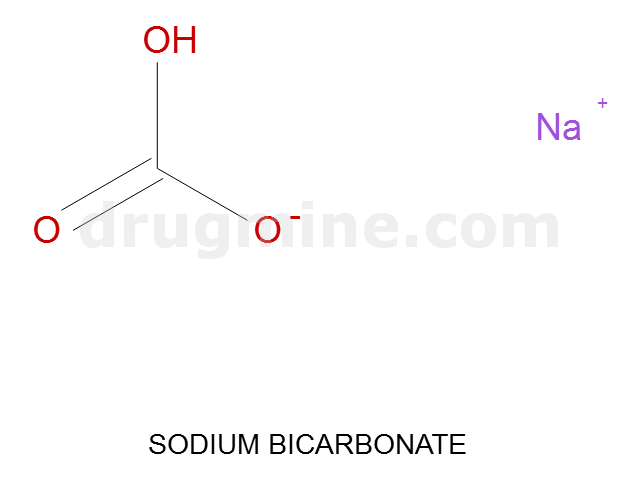

Name: SODIUM BICARBONATE
ID :
MW: 61
Number of atoms: 4
Molecular_Formula: CHO3
Alogp: -1.261
Indication class : Alkalizer (systemic); Replenisher (electrolyte)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
sodium bicarbonate containing products summary
There are in total 73 different products containing the active ingredient sodium bicarbonate. From the 73 drug products, 30 have been discontinued.Product id = 13628
Application Number = 18469
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = BSS PLUS
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.154MG/ML;0.92MG/ML;0.184MG/ML;0.2MG/ML;0.38MG/ML;2.1MG/ML;7.14MG/ML;0.42MG/ML
----
Product id = 5475
Application Number = 18509
Date of Application = 7,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = BAROS
Route/format = ORAL / GRANULE, EFFERVESCENT
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode =
Rld = No
Strength = 460MG/GM;420MG/GM
----
Product id = 4847
Application Number = 18983
Date of Application = 26,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = COLYTE
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 227.1GM/PACKET;2.82GM/PACKET;6.36GM/PACKET;5.53GM/PACKET;21.5GM/PACKET
----
Product id = 4846
Application Number = 18983
Date of Application = 26,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = COLYTE
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 120GM/PACKET;1.49GM/PACKET;3.36GM/PACKET;2.92GM/PACKET;11.36GM/PACKET
----
Product id = 4850
Application Number = 18983
Date of Application = 26,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = COLYTE
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 006
Tecode =
Rld = No
Strength = 360GM/PACKET;4.47GM/PACKET;10.08GM/PACKET;8.76GM/PACKET;34.08GM/PACKET
----
Product id = 4849
Application Number = 18983
Date of Application = 12,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = COLYTE
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 007
Tecode =
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 4852
Application Number = 18983
Date of Application = 14,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = COLYTE-FLAVORED
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 008
Tecode =
Rld = No
Strength = 227.1GM/BOT;2.82GM/BOT;6.36GM/BOT;5.53GM/BOT;21.5GM/BOT
----
Product id = 4853
Application Number = 18983
Date of Application = 14,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = COLYTE-FLAVORED
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 009
Tecode =
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 4848
Application Number = 18983
Date of Application = 31,, Jan, 1989
RX/OTC/DISCN = RX
Tradename = COLYTE
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 010
Tecode =
Rld = No
Strength = 227.1GM/BOT;2.82GM/BOT;6.36GM/BOT;5.53GM/BOT;21.5GM/BOT
----
Product id = 4851
Application Number = 18983
Date of Application = 8,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = COLYTE WITH FLAVOR PACKS
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 012
Tecode =
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 4862
Application Number = 19011
Date of Application = 13,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = GOLYTELY
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 4861
Application Number = 19011
Date of Application = 2,, Jun, 1992
RX/OTC/DISCN = RX
Tradename = GOLYTELY
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 227.1GM/PACKET;2.82GM/PACKET;6.36GM/PACKET;5.53GM/PACKET;21.5GM/PACKET
----
Product id = 13963
Application Number = 19284
Date of Application = 30,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = OCL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 6GM/100ML;75MG/100ML;168MG/100ML;146MG/100ML;1.29GM/100ML
----
Product id = 10778
Application Number = 19443
Date of Application = 3,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = SODIUM BICARBONATE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 0.9MEQ/ML
----
Product id = 10779
Application Number = 19443
Date of Application = 3,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = SODIUM BICARBONATE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBOTT
ProductNo = 002
Tecode =
Rld = No
Strength = 1MEQ/ML
----
Product id = 4875
Application Number = 19797
Date of Application = 22,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = NULYTELY
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 4876
Application Number = 19797
Date of Application = 18,, Nov, 1994
RX/OTC/DISCN = RX
Tradename = NULYTELY-FLAVORED
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 002
Tecode = AA
Rld = Yes
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 13629
Application Number = 20079
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = ENDOSOL EXTRA
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.154MG/ML;0.92MG/ML;0.184MG/ML;0.2MG/ML;0.38MG/ML;2.1MG/ML;7.14MG/ML;0.42MG/ML
----
Product id = 11606
Application Number = 20577
Date of Application = 27,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = ELLIOTTS B SOLUTION
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = LUKARE MEDICAL LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.2MG/ML;0.8MG/ML;0.3MG/ML;0.3MG/ML;1.9MG/ML;7.3MG/ML;0.2MG/ML
----
Product id = 4805
Application Number = 21551
Date of Application = 16,, Jul, 2010
RX/OTC/DISCN = DISCN
Tradename = HALFLYTELY
Route/format = ORAL / FOR SOLUTION, TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG,N/A;N/A,210GM;N/A,0.74GM;N/A,2.86GM;N/A,5.6GM
----
Product id = 5312
Application Number = 21636
Date of Application = 15,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = ZEGERID
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/PACKET;1.68GM/PACKET
----
Product id = 5313
Application Number = 21636
Date of Application = 21,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = ZEGERID
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 40MG/PACKET;1.68GM/PACKET
----
Product id = 10452
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRISMASOL BK 0/3.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5.15GM/1000ML;N/A/1000ML;5.4GM/1000ML;2.03GM/1000ML;N/A/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10444
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRISMASOL BGK 2/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 002
Tecode =
Rld = Yes
Strength = N/A/1000ML;20GM/1000ML;5.4GM/1000ML;2.03GM/1000ML;0.157GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10445
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRISMASOL BGK 2/3.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 003
Tecode =
Rld = Yes
Strength = 5.15GM/1000ML;20GM/1000ML;5.4GM/1000ML;2.03GM/1000ML;0.157GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10448
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRISMASOL BGK 4/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 004
Tecode =
Rld = Yes
Strength = 3.68GM/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.314GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10446
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = DISCN
Tradename = PRISMASOL BGK 4/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 005
Tecode =
Rld = No
Strength = N/A/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.314GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10443
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRISMASOL BGK 0/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 006
Tecode =
Rld = Yes
Strength = 3.68GM/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;N/A/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10450
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = DISCN
Tradename = PRISMASOL BK 0/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 007
Tecode =
Rld = No
Strength = N/A/1000ML;N/A/1000ML;5.4GM/1000ML;3.05GM/1000ML;N/A/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10449
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = DISCN
Tradename = PRISMASOL BGK 4/3.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 008
Tecode =
Rld = No
Strength = 5.15GM/1000ML;20GM/1000ML;5.4GM/1000ML;2.03GM/1000ML;0.314GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10453
Application Number = 21703
Date of Application = 25,, Oct, 2006
RX/OTC/DISCN = DISCN
Tradename = PRISMASOL BK 4/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 009
Tecode =
Rld = No
Strength = 3.68GM/1000ML;N/A/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.314GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10439
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = PRISMASOL B22GK 2/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 010
Tecode =
Rld = Yes
Strength = N/A/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.157GM/1000ML;2.21GM/1000ML;7.07GM/1000ML (5000ML)
----
Product id = 10441
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = PRISMASOL B22GK 4/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 011
Tecode =
Rld = Yes
Strength = N/A/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.314GM/1000ML;2.21GM/1000ML;7.07GM/1000ML (5000ML)
----
Product id = 10440
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = DISCN
Tradename = PRISMASOL B22GK 2/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 012
Tecode =
Rld = No
Strength = 3.68GM/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.157GM/1000ML;2.21GM/1000ML;7.07GM/1000ML (5000ML)
----
Product id = 10442
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = PRISMASOL B22GK 4/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 013
Tecode =
Rld = Yes
Strength = 3.68GM/1000ML;20GM/1000ML;5.4GM/1000ML;3.05GM/1000ML;0.314GM/1000ML;2.21GM/1000ML;7.07GM/1000ML (5000ML)
----
Product id = 10451
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = PRISMASOL BK 0/0/1.2 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 014
Tecode =
Rld = Yes
Strength = N/A/1000ML;N/A/1000ML;5.4GM/1000ML;2.44GM/1000ML;N/A/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 10447
Application Number = 21703
Date of Application = 10,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = PRISMASOL BGK 4/0/1.2 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO RENAL PRODS
ProductNo = 015
Tecode =
Rld = Yes
Strength = N/A/1000ML;20GM/1000ML;5.4GM/1000ML;2.44GM/1000ML;0.314GM/1000ML;3.09GM/1000ML;6.46GM/1000ML (5000ML)
----
Product id = 3804
Application Number = 21849
Date of Application = 27,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = ZEGERID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG;1.1GM
----
Product id = 3805
Application Number = 21849
Date of Application = 27,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = ZEGERID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 40MG;1.1GM
----
Product id = 15377
Application Number = 21850
Date of Application = 24,, Mar, 2006
RX/OTC/DISCN = DISCN
Tradename = ZEGERID
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = SANTARUS
ProductNo = 001
Tecode =
Rld = No
Strength = 700MG;20MG;600MG
----
Product id = 15378
Application Number = 21850
Date of Application = 24,, Mar, 2006
RX/OTC/DISCN = DISCN
Tradename = ZEGERID
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = SANTARUS
ProductNo = 002
Tecode =
Rld = No
Strength = 700MG;40MG;600MG
----
Product id = 13438
Application Number = 21910
Date of Application = 26,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = NORMOCARB HF 25
Route/format = INJECTION / SOLUTION
Application Type = N
Applicant Name = DIALYSIS SUPS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.21GM/100ML;2.8GM/100ML;9.07GM/100ML
----
Product id = 13439
Application Number = 21910
Date of Application = 26,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = NORMOCARB HF 35
Route/format = INJECTION / SOLUTION
Application Type = N
Applicant Name = DIALYSIS SUPS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 0.21GM/100ML;3.97GM/100ML;8.3GM/100ML
----
Product id = 13640
Application Number = 22193
Date of Application = 24,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = NAVSTEL
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.154MG/ML;0.92MG/ML;0.2MG/ML;0.184MG/ML;0.38MG/ML;2.1MG/ML;7.14MG/ML;0.42MG/ML
----
Product id = 3806
Application Number = 22281
Date of Application = 1,, Dec, 2009
RX/OTC/DISCN = OTC
Tradename = ZEGERID OTC
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MSD CONSUMER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG;1.1GM
----
Product id = 5314
Application Number = 22283
Date of Application = 17,, Jun, 2013
RX/OTC/DISCN = OTC
Tradename = ZEGERID OTC
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = MSD CONSUMER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG/PACKET;1.68GM/PACKET
----
Product id = 24105
Application Number = 22456
Date of Application = 4,, Dec, 2009
RX/OTC/DISCN = DISCN
Tradename = MAGNESIUM HYDROXIDE AND OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANTARUS
ProductNo = 001
Tecode =
Rld = No
Strength = 343MG;20MG;750MG
----
Product id = 24106
Application Number = 22456
Date of Application = 4,, Dec, 2009
RX/OTC/DISCN = DISCN
Tradename = MAGNESIUM HYDROXIDE AND OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANTARUS
ProductNo = 002
Tecode =
Rld = No
Strength = 343MG;40MG;750MG
----
Product id = 5206
Application Number = 71278
Date of Application = 21,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = E-Z-EM PREP LYTE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = E Z EM
ProductNo = 001
Tecode =
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 5192
Application Number = 71320
Date of Application = 20,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = COLOVAGE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = DYNAPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 227.1GM/PACKET;2.82GM/PACKET;6.36GM/PACKET;5.53GM/PACKET;21.5GM/PACKET
----
Product id = 5220
Application Number = 72319
Date of Application = 23,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = GLYCOPREP
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = GOLDLINE
ProductNo = 001
Tecode =
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 5241
Application Number = 73098
Date of Application = 31,, Aug, 1993
RX/OTC/DISCN = DISCN
Tradename = PEG-LYTE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 5191
Application Number = 73428
Date of Application = 28,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = CO-LAV
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 5221
Application Number = 73433
Date of Application = 28,, Apr, 1992
RX/OTC/DISCN = DISCN
Tradename = GO-EVAC
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 4936
Application Number = 76491
Date of Application = 5,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = TRILYTE
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 10776
Application Number = 77394
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = SODIUM BICARBONATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.9MEQ/ML
----
Product id = 10777
Application Number = 77394
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = SODIUM BICARBONATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1MEQ/ML
----
Product id = 2706
Application Number = 78966
Date of Application = 25,, May, 2010
RX/OTC/DISCN = RX
Tradename = OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG;1.1GM
----
Product id = 2707
Application Number = 78966
Date of Application = 25,, May, 2010
RX/OTC/DISCN = RX
Tradename = OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG;1.1GM
----
Product id = 5232
Application Number = 79182
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = DISCN
Tradename = OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/PACKET;1.68GM/PACKET
----
Product id = 5233
Application Number = 79182
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = DISCN
Tradename = OMEPRAZOLE AND SODIUM BICARBONATE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG/PACKET;1.68GM/PACKET
----
Product id = 4866
Application Number = 79232
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = LAX-LYTE WITH FLAVOR PACKS
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 4884
Application Number = 90019
Date of Application = 27,, May, 2009
RX/OTC/DISCN = RX
Tradename = PEG-3350;POTASSIUM CHLORIDE;SODIUM BICARBONATE;SODIUM CHLORIDE
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 4882
Application Number = 90186
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = PEG 3350 AND ELECTROLYTES
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 4881
Application Number = 90231
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = PEG 3350 AND ELECTROLYTES
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 4883
Application Number = 90409
Date of Application = 2,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = PEG-3350;POTASSIUM CHLORIDE;SODIUM BICARBONATE;SODIUM CHLORIDE
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AA
Rld = No
Strength = 420GM/BOT;1.48GM/BOT;5.72GM/BOT;11.2GM/BOT
----
Product id = 4925
Application Number = 90712
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = DISCN
Tradename = POLYETHYLENE GLYCOL 3350 AND ELECTROLYTES
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 240GM/BOT;2.98GM/BOT;6.72GM/BOT;5.84GM/BOT;22.72GM/BOT
----
Product id = 4837
Application Number = 90769
Date of Application = 7,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = CLENZ-LYTE
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 236GM/BOT;2.97GM/BOT;6.74GM/BOT;5.86GM/BOT;22.74GM/BOT
----
Product id = 4880
Application Number = 90928
Date of Application = 28,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = PEG 3350 AND ELECTROLYTES
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AA
Rld = No
Strength = 236GM;2.97GM;6.74GM;5.86GM;22.74GM
----
Product id = 4806
Application Number = 202217
Date of Application = 20,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = PEG-3350, SODIUM CHLORIDE, SODIUM BICARBONATE, POTASSIUM CHLORIDE AND BISACODYL
Route/format = ORAL / FOR SOLUTION, TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG,N/A;N/A,210GM;N/A,0.74GM;N/A,2.86GM;N/A,5.6GM
----
Product id = 14045
Application Number = 203595
Date of Application = 18,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = SUCLEAR
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = BRAINTREE LABS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1.6GM/BOT,3.13GM/BOT,17.5GM/BOT,N/A,N/A,N/A,N/A;N/A,N/A,N/A,210GM,0.74GM,2.86GM,5.6GM
----
Product id = 10128
Application Number = 207026
Date of Application = 13,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = PHOXILLUM BK 4/2.5 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO LUNDIA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 3.68GM/1000ML;3.05GM/1000ML;0.314GM/1000ML ;3.09GM/1000ML;6.34GM/1000ML;0.187GM/1000ML (5000ML)
----
Product id = 10127
Application Number = 207026
Date of Application = 13,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = PHOXILLUM B22K 4/0 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GAMBRO LUNDIA
ProductNo = 002
Tecode =
Rld = Yes
Strength = N/A/1000ML;3.05GM/1000ML;0.314GM/1000ML;2.21GM/1000ML;6.95GM/1000ML;0.187GM/1000ML (5000ML)
----