tacrine-hydrochloride
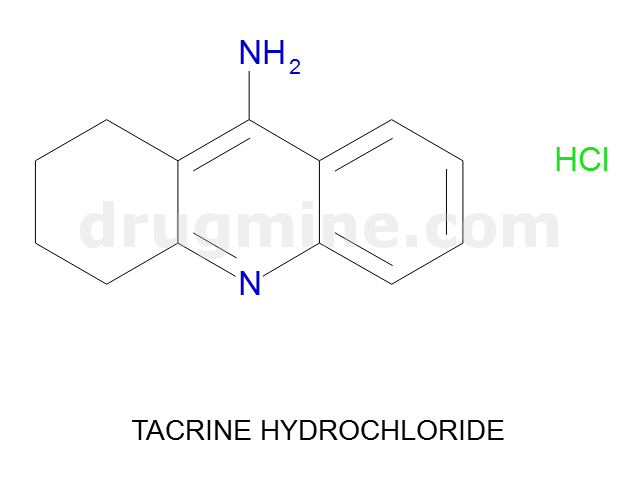

Name: TACRINE HYDROCHLORIDE
ID :
MW: 198
Number of atoms: 15
Molecular_Formula: C13H14N2
Alogp: 2.79
Indication class : Cognition Adjuvant; Dementia Symptoms Treatment Adjunct
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
tacrine hydrochloride containing products summary
There are in total 4 different products containing the active ingredient tacrine hydrochloride. From the 4 drug products, 4 have been discontinued.Product id = 1518
Application Number = 20070
Date of Application = 9,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = COGNEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1519
Application Number = 20070
Date of Application = 9,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = COGNEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1520
Application Number = 20070
Date of Application = 9,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = COGNEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE
----
Product id = 1521
Application Number = 20070
Date of Application = 9,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = COGNEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----