timolol-maleate
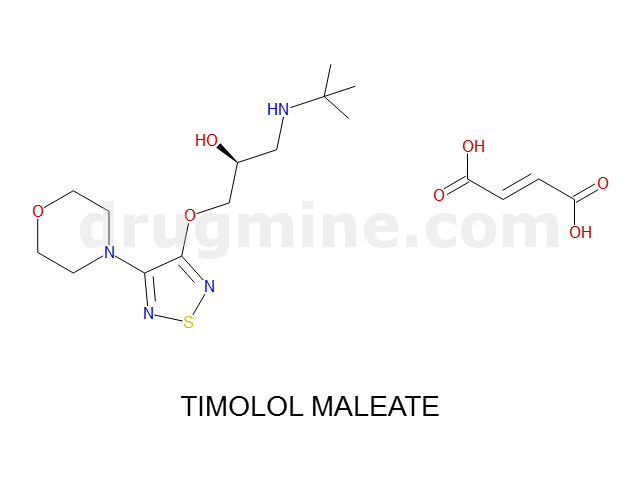

Name: TIMOLOL MALEATE
ID :
MW: 316
Number of atoms: 21
Molecular_Formula: C13H24N4O3S
Alogp: 1.127
Indication class : Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
timolol maleate containing products summary
There are in total 66 different products containing the active ingredient timolol maleate. From the 66 drug products, 29 have been discontinued.Product id = 18605
Application Number = 18017
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BLOCADREN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18606
Application Number = 18017
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BLOCADREN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18607
Application Number = 18017
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BLOCADREN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28716
Application Number = 18061
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TIMOLIDE 10-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;10MG
----
Product id = 13163
Application Number = 18086
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TIMOPTIC
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ATON
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = EQ 0.25% BASE
----
Product id = 13164
Application Number = 18086
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TIMOPTIC
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ATON
ProductNo = 002
Tecode = AT
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 13165
Application Number = 19463
Date of Application = 5,, Nov, 1986
RX/OTC/DISCN = RX
Tradename = TIMOPTIC IN OCUDOSE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ATON
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.25% BASE
----
Product id = 13166
Application Number = 19463
Date of Application = 5,, Nov, 1986
RX/OTC/DISCN = RX
Tradename = TIMOPTIC IN OCUDOSE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ATON
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 12834
Application Number = 20330
Date of Application = 4,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = TIMOPTIC-XE
Route/format = OPHTHALMIC / SOLUTION, GEL FORMING/DROPS
Application Type = N
Applicant Name = VALEANT PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.25% BASE
----
Product id = 12835
Application Number = 20330
Date of Application = 4,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = TIMOPTIC-XE
Route/format = OPHTHALMIC / SOLUTION, GEL FORMING/DROPS
Application Type = N
Applicant Name = VALEANT PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 12911
Application Number = 20869
Date of Application = 7,, Apr, 1998
RX/OTC/DISCN = RX
Tradename = COSOPT
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12832
Application Number = 20963
Date of Application = 21,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION, GEL FORMING/DROPS
Application Type = N
Applicant Name = ALCON RES LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 12833
Application Number = 20963
Date of Application = 21,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION, GEL FORMING/DROPS
Application Type = N
Applicant Name = ALCON RES LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 12910
Application Number = 21398
Date of Application = 30,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = COMBIGAN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.2%;EQ 0.5% BASE
----
Product id = 12990
Application Number = 21516
Date of Application = 4,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = ISTALOL
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = BT
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 28729
Application Number = 72001
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28730
Application Number = 72002
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28731
Application Number = 72003
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28732
Application Number = 72269
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28734
Application Number = 72270
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28736
Application Number = 72271
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28720
Application Number = 72466
Date of Application = 19,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28721
Application Number = 72467
Date of Application = 19,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28722
Application Number = 72468
Date of Application = 19,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28723
Application Number = 72550
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28724
Application Number = 72551
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28725
Application Number = 72552
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28726
Application Number = 72648
Date of Application = 16,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28727
Application Number = 72649
Date of Application = 16,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28728
Application Number = 72650
Date of Application = 16,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 28719
Application Number = 72668
Date of Application = 8,, Jun, 1990
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG
----
Product id = 28717
Application Number = 72668
Date of Application = 8,, Jun, 1990
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28718
Application Number = 72668
Date of Application = 8,, Jun, 1990
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28733
Application Number = 72917
Date of Application = 31,, Jul, 1991
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28735
Application Number = 72918
Date of Application = 31,, Jul, 1991
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28737
Application Number = 72919
Date of Application = 31,, Jul, 1991
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 13148
Application Number = 74261
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON RES LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13149
Application Number = 74262
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON RES LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13144
Application Number = 74465
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13146
Application Number = 74466
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13145
Application Number = 74515
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13147
Application Number = 74516
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13156
Application Number = 74667
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13157
Application Number = 74668
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13159
Application Number = 74746
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = PACIFIC PHARMA
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13160
Application Number = 74747
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = PACIFIC PHARMA
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13153
Application Number = 74776
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13152
Application Number = 74778
Date of Application = 25,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13158
Application Number = 75163
Date of Application = 10,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13150
Application Number = 75411
Date of Application = 8,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13151
Application Number = 75412
Date of Application = 8,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13154
Application Number = 77259
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FDC LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13155
Application Number = 77259
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FDC LTD
ProductNo = 002
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 12952
Application Number = 77847
Date of Application = 28,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12950
Application Number = 78201
Date of Application = 28,, Oct, 2008
RX/OTC/DISCN = DISCN
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12955
Application Number = 78704
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12954
Application Number = 78749
Date of Application = 6,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 13161
Application Number = 78771
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13162
Application Number = 78771
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 12951
Application Number = 90037
Date of Application = 14,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12949
Application Number = 90604
Date of Application = 18,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON RES
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12957
Application Number = 91180
Date of Application = 4,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ZACH SYSTEMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12953
Application Number = 201998
Date of Application = 17,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = LANNETT HOLDINGS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12956
Application Number = 202054
Date of Application = 3,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12912
Application Number = 202667
Date of Application = 1,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = COSOPT PF
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 2% BASE;EQ 0.5% BASE
----
Product id = 12948
Application Number = 203058
Date of Application = 22,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 2% BASE;EQ 0.5% BASE
----