tramadol-hydrochloride
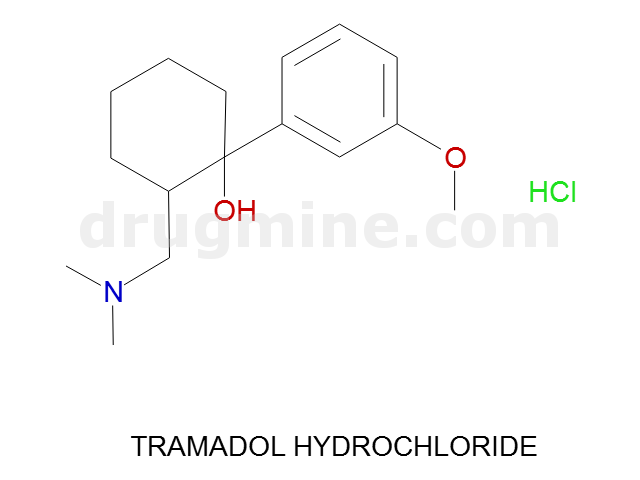
Name: TRAMADOL HYDROCHLORIDE
ID :
MW: 263
Number of atoms: 19
Molecular_Formula: C16H25NO2
Alogp: 2.7
Indication class : Analgesic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
tramadol hydrochloride containing products summary
There are in total 62 different products containing the active ingredient tramadol hydrochloride. From the 62 drug products, 11 have been discontinued.Product id = 29283
Application Number = 20281
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = ULTRAM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29282
Application Number = 20281
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = ULTRAM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 50MG
----
Product id = 29279
Application Number = 21123
Date of Application = 15,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = ULTRACET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 325MG;37.5MG
----
Product id = 16736
Application Number = 21692
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ULTRAM ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 001
Tecode = AB1
Rld = Yes
Strength = 100MG
----
Product id = 16737
Application Number = 21692
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ULTRAM ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 200MG
----
Product id = 16738
Application Number = 21692
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ULTRAM ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 300MG
----
Product id = 17099
Application Number = 21693
Date of Application = 5,, May, 2005
RX/OTC/DISCN = DISCN
Tradename = RYBIX ODT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 16618
Application Number = 21745
Date of Application = 30,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RYZOLT
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 16619
Application Number = 21745
Date of Application = 30,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RYZOLT
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 16620
Application Number = 21745
Date of Application = 30,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RYZOLT
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 377
Application Number = 22370
Date of Application = 7,, May, 2010
RX/OTC/DISCN = RX
Tradename = CONZIP
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG
----
Product id = 379
Application Number = 22370
Date of Application = 7,, May, 2010
RX/OTC/DISCN = RX
Tradename = CONZIP
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 380
Application Number = 22370
Date of Application = 7,, May, 2010
RX/OTC/DISCN = RX
Tradename = CONZIP
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 378
Application Number = 22370
Date of Application = 1,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = CONZIP
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 150MG
----
Product id = 28981
Application Number = 75960
Date of Application = 19,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28999
Application Number = 75962
Date of Application = 24,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28989
Application Number = 75963
Date of Application = 3,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28997
Application Number = 75964
Date of Application = 19,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28996
Application Number = 75968
Date of Application = 25,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28985
Application Number = 75974
Date of Application = 12,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASTA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28998
Application Number = 75977
Date of Application = 19,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28993
Application Number = 75980
Date of Application = 21,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28984
Application Number = 75981
Date of Application = 10,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28995
Application Number = 75982
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28990
Application Number = 75983
Date of Application = 25,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28992
Application Number = 75986
Date of Application = 21,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28983
Application Number = 76003
Date of Application = 20,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28991
Application Number = 76100
Date of Application = 20,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29006
Application Number = 76475
Date of Application = 21,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 29008
Application Number = 76914
Date of Application = 26,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 325MG;37.5MG
----
Product id = 29007
Application Number = 77184
Date of Application = 16,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 29005
Application Number = 77858
Date of Application = 26,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 29003
Application Number = 78778
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 16715
Application Number = 78783
Date of Application = 13,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 100MG
----
Product id = 16716
Application Number = 78783
Date of Application = 13,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM INC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 200MG
----
Product id = 16717
Application Number = 78783
Date of Application = 20,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM INC
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 300MG
----
Product id = 28994
Application Number = 78935
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29000
Application Number = 90404
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29009
Application Number = 90460
Date of Application = 6,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 29002
Application Number = 90485
Date of Application = 9,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 28987
Application Number = 91498
Date of Application = 29,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CSPC OUYI PHARM CO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 16718
Application Number = 91607
Date of Application = 30,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB2
Rld = Yes
Strength = 100MG
----
Product id = 16720
Application Number = 91607
Date of Application = 30,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 200MG
----
Product id = 16722
Application Number = 91607
Date of Application = 30,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 300MG
----
Product id = 16706
Application Number = 91609
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 100MG
----
Product id = 16707
Application Number = 91609
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 200MG
----
Product id = 16708
Application Number = 91609
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 300MG
----
Product id = 16709
Application Number = 200491
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 100MG
----
Product id = 16710
Application Number = 200491
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 200MG
----
Product id = 16711
Application Number = 200491
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 300MG
----
Product id = 16712
Application Number = 200503
Date of Application = 29,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 100MG
----
Product id = 16713
Application Number = 200503
Date of Application = 29,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 200MG
----
Product id = 16714
Application Number = 200503
Date of Application = 29,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 300MG
----
Product id = 16719
Application Number = 201384
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 100MG
----
Product id = 16721
Application Number = 201384
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 200MG
----
Product id = 16723
Application Number = 201384
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 300MG
----
Product id = 29004
Application Number = 201952
Date of Application = 14,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 28988
Application Number = 201973
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28982
Application Number = 202075
Date of Application = 28,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALLIED PHARMA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29001
Application Number = 202076
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALLIED PHARMA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;37.5MG
----
Product id = 28980
Application Number = 202390
Date of Application = 16,, May, 2013
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28986
Application Number = 203494
Date of Application = 31,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = TRAMADOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----