trifluoperazine-hydrochloride
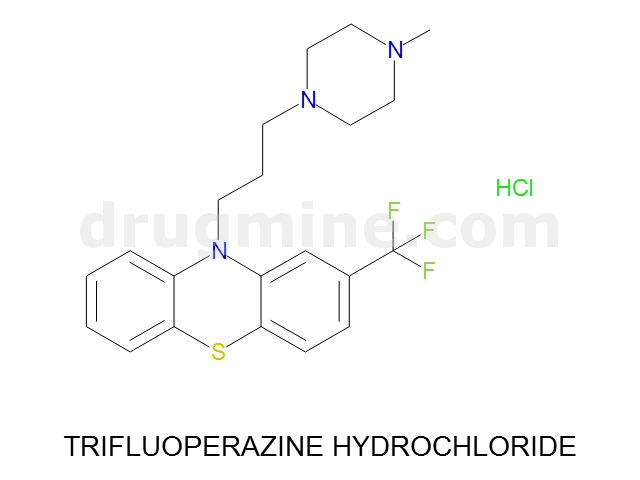
Name: TRIFLUOPERAZINE HYDROCHLORIDE
ID :
MW: 407
Number of atoms: 28
Molecular_Formula: C21H24F3N3S
Alogp: 4.975
Indication class : Antipsychotic; Sedative-Hypnotic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
trifluoperazine hydrochloride containing products summary
There are in total 32 different products containing the active ingredient trifluoperazine hydrochloride. From the 32 drug products, 24 have been discontinued.Product id = 28245
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 28246
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28247
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 28248
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 10875
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 3973
Application Number = 11552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STELAZINE
Route/format = ORAL / CONCENTRATE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 29183
Application Number = 40153
Date of Application = 25,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29185
Application Number = 40153
Date of Application = 25,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29187
Application Number = 40153
Date of Application = 25,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29189
Application Number = 40153
Date of Application = 25,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 29179
Application Number = 40209
Date of Application = 7,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29180
Application Number = 40209
Date of Application = 7,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29181
Application Number = 40209
Date of Application = 7,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29182
Application Number = 40209
Date of Application = 7,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE
----
Product id = 29184
Application Number = 85785
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29186
Application Number = 85786
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 3997
Application Number = 85787
Date of Application = 15,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 29190
Application Number = 85788
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 29188
Application Number = 85789
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29193
Application Number = 85973
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29191
Application Number = 85975
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29192
Application Number = 85976
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29177
Application Number = 87328
Date of Application = 19,, Nov, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29175
Application Number = 87612
Date of Application = 19,, Nov, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29176
Application Number = 87613
Date of Application = 19,, Nov, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29178
Application Number = 87614
Date of Application = 19,, Nov, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 3998
Application Number = 88143
Date of Application = 26,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 29194
Application Number = 88710
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 29171
Application Number = 88967
Date of Application = 23,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 29172
Application Number = 88968
Date of Application = 23,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29173
Application Number = 88969
Date of Application = 23,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 29174
Application Number = 88970
Date of Application = 23,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = TRIFLUOPERAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----