trospium-chloride
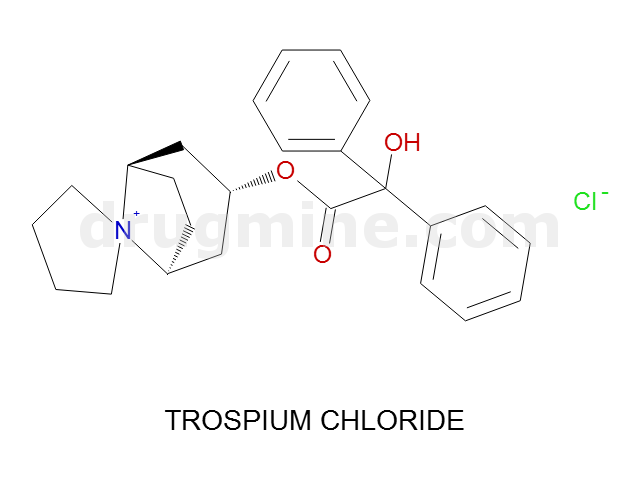


Name: TROSPIUM CHLORIDE
ID :
MW: 393
Number of atoms: 29
Molecular_Formula: C25H30NO3
Alogp: 2.349
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
trospium chloride containing products summary
There are in total 7 different products containing the active ingredient trospium chloride. From the 7 drug products, zero have been discontinued.Product id = 27827
Application Number = 21595
Date of Application = 28,, May, 2004
RX/OTC/DISCN = RX
Tradename = SANCTURA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 742
Application Number = 22103
Date of Application = 3,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = SANCTURA XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 60MG
----
Product id = 812
Application Number = 91289
Date of Application = 12,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = TROSPIUM CHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 29252
Application Number = 91513
Date of Application = 6,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TROSPIUM CHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 29254
Application Number = 91573
Date of Application = 17,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = TROSPIUM CHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 29253
Application Number = 91575
Date of Application = 13,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = TROSPIUM CHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 813
Application Number = 201291
Date of Application = 24,, May, 2013
RX/OTC/DISCN = RX
Tradename = TROSPIUM CHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----