venlafaxine-hydrochloride
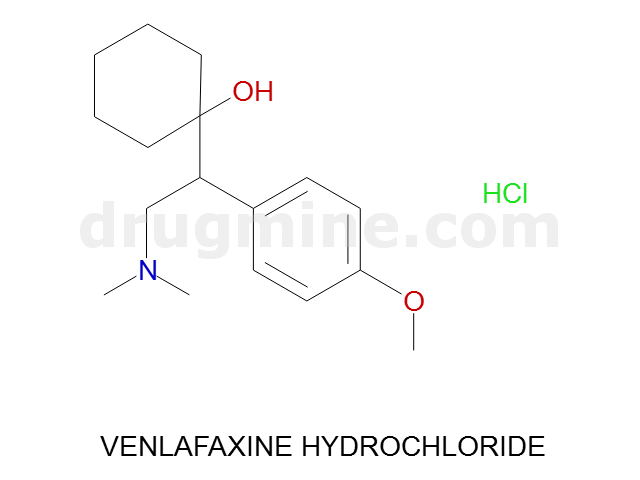

Name: VENLAFAXINE HYDROCHLORIDE
ID :
MW: 277
Number of atoms: 20
Molecular_Formula: C17H27NO2
Alogp: 3.022
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
venlafaxine hydrochloride containing products summary
There are in total 107 different products containing the active ingredient venlafaxine hydrochloride. From the 107 drug products, 17 have been discontinued.Product id = 20780
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20781
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 25MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20783
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 50MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20784
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 75MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20785
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 100MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20782
Application Number = 20151
Date of Application = 28,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 37.5MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 497
Application Number = 20699
Date of Application = 20,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = EFFEXOR XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 498
Application Number = 20699
Date of Application = 20,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = EFFEXOR XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 499
Application Number = 20699
Date of Application = 20,, Oct, 1997
RX/OTC/DISCN = DISCN
Tradename = EFFEXOR XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 500
Application Number = 20699
Date of Application = 20,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = EFFEXOR XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 150MG BASE
----
Product id = 16746
Application Number = 22104
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = OSMOTICA PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 16747
Application Number = 22104
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = OSMOTICA PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 16748
Application Number = 22104
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = OSMOTICA PHARM
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 150MG BASE
----
Product id = 16749
Application Number = 22104
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = OSMOTICA PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 225MG BASE
----
Product id = 832
Application Number = 76565
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 833
Application Number = 76565
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 834
Application Number = 76565
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 29514
Application Number = 76690
Date of Application = 3,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29515
Application Number = 76690
Date of Application = 3,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29516
Application Number = 76690
Date of Application = 3,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 50MG BASE
----
Product id = 29517
Application Number = 76690
Date of Application = 3,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29518
Application Number = 76690
Date of Application = 3,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29494
Application Number = 77166
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29495
Application Number = 77166
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29496
Application Number = 77166
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29497
Application Number = 77166
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29498
Application Number = 77166
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29504
Application Number = 77515
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29505
Application Number = 77515
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29506
Application Number = 77515
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29507
Application Number = 77515
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29508
Application Number = 77515
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29524
Application Number = 77653
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29525
Application Number = 77653
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29526
Application Number = 77653
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29527
Application Number = 77653
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29528
Application Number = 77653
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 817
Application Number = 78087
Date of Application = 16,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 818
Application Number = 78087
Date of Application = 16,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 819
Application Number = 78087
Date of Application = 16,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 29484
Application Number = 78301
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29485
Application Number = 78301
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29486
Application Number = 78301
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29487
Application Number = 78301
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29488
Application Number = 78301
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 823
Application Number = 78421
Date of Application = 6,, May, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 824
Application Number = 78421
Date of Application = 6,, May, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 825
Application Number = 78421
Date of Application = 6,, May, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 29499
Application Number = 78517
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29500
Application Number = 78517
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29501
Application Number = 78517
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29502
Application Number = 78517
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29503
Application Number = 78517
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29489
Application Number = 78554
Date of Application = 9,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29490
Application Number = 78554
Date of Application = 9,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29491
Application Number = 78554
Date of Application = 9,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29492
Application Number = 78554
Date of Application = 9,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29493
Application Number = 78554
Date of Application = 9,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29509
Application Number = 78627
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29510
Application Number = 78627
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29511
Application Number = 78627
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29512
Application Number = 78627
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29513
Application Number = 78627
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 826
Application Number = 78789
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 827
Application Number = 78789
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 828
Application Number = 78789
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 841
Application Number = 78865
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 842
Application Number = 78865
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 843
Application Number = 78865
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 29469
Application Number = 78932
Date of Application = 14,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29470
Application Number = 78932
Date of Application = 14,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29471
Application Number = 78932
Date of Application = 14,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29472
Application Number = 78932
Date of Application = 14,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29473
Application Number = 78932
Date of Application = 14,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29474
Application Number = 79098
Date of Application = 11,, May, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29475
Application Number = 79098
Date of Application = 11,, May, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29476
Application Number = 79098
Date of Application = 11,, May, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29477
Application Number = 79098
Date of Application = 11,, May, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29478
Application Number = 79098
Date of Application = 11,, May, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 29519
Application Number = 90027
Date of Application = 4,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29520
Application Number = 90027
Date of Application = 4,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29521
Application Number = 90027
Date of Application = 4,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29522
Application Number = 90027
Date of Application = 4,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29523
Application Number = 90027
Date of Application = 4,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 838
Application Number = 90071
Date of Application = 15,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 839
Application Number = 90071
Date of Application = 15,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 840
Application Number = 90071
Date of Application = 15,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 844
Application Number = 90174
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 845
Application Number = 90174
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 846
Application Number = 90174
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 29479
Application Number = 90555
Date of Application = 7,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29480
Application Number = 90555
Date of Application = 7,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 29481
Application Number = 90555
Date of Application = 7,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29482
Application Number = 90555
Date of Application = 7,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 29483
Application Number = 90555
Date of Application = 7,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 835
Application Number = 90899
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 836
Application Number = 90899
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 837
Application Number = 90899
Date of Application = 1,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 829
Application Number = 91123
Date of Application = 11,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 830
Application Number = 91123
Date of Application = 11,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 831
Application Number = 91123
Date of Application = 11,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 16750
Application Number = 91272
Date of Application = 18,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 16751
Application Number = 91272
Date of Application = 18,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 16752
Application Number = 91272
Date of Application = 18,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 820
Application Number = 200834
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 821
Application Number = 200834
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 75MG BASE
----
Product id = 822
Application Number = 200834
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = VENLAFAXINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----