vincristine-sulfate
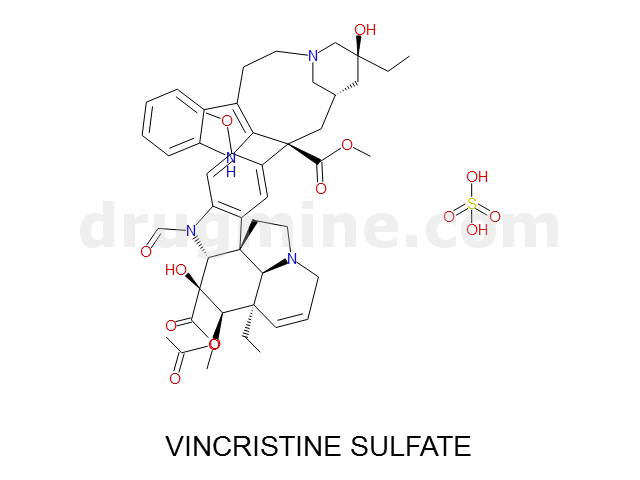

Name: VINCRISTINE SULFATE
ID :
MW: 825
Number of atoms: 60
Molecular_Formula: C46H56N4O10
Alogp: 3.972
Indication class : Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
vincristine sulfate containing products summary
There are in total 15 different products containing the active ingredient vincristine sulfate. From the 15 drug products, 12 have been discontinued.Product id = 9817
Application Number = 14103
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ONCOVIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/VIAL
----
Product id = 9819
Application Number = 14103
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ONCOVIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 9818
Application Number = 14103
Date of Application = 7,, Mar, 1984
RX/OTC/DISCN = DISCN
Tradename = ONCOVIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 11384
Application Number = 70411
Date of Application = 10,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 11382
Application Number = 70867
Date of Application = 12,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = VINCREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 11383
Application Number = 70873
Date of Application = 19,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABIC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 11381
Application Number = 71426
Date of Application = 17,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VINCASAR PFS
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 11390
Application Number = 71484
Date of Application = 19,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = VINCRISTINE SULFATE PFS
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 11387
Application Number = 71559
Date of Application = 11,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/VIAL
----
Product id = 11388
Application Number = 71560
Date of Application = 11,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/VIAL
----
Product id = 11389
Application Number = 71561
Date of Application = 11,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 11391
Application Number = 75493
Date of Application = 1,, Sep, 1999
RX/OTC/DISCN = RX
Tradename = VINCRISTINE SULFATE PFS
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 11385
Application Number = 76296
Date of Application = 20,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 11386
Application Number = 76401
Date of Application = 28,, Oct, 2003
RX/OTC/DISCN = DISCN
Tradename = VINCRISTINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 5566
Application Number = 202497
Date of Application = 9,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = MARQIBO KIT
Route/format = INTRAVENOUS / INJECTABLE, LIPOSOMAL
Application Type = N
Applicant Name = TALON THERAP
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG/5ML (1MG/ML)
----