ziprasidone-hydrochloride
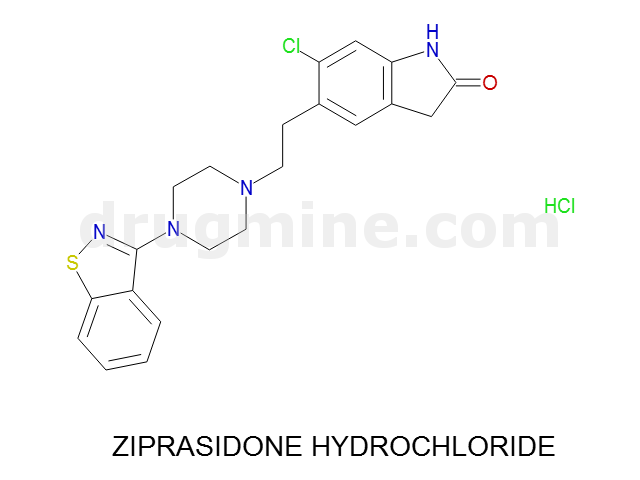

Name: ZIPRASIDONE HYDROCHLORIDE
ID :
MW: 413
Number of atoms: 28
Molecular_Formula: C21H21ClN4OS
Alogp: 4.259
Indication class : Antipsychotic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ziprasidone hydrochloride containing products summary
There are in total 29 different products containing the active ingredient ziprasidone hydrochloride. From the 29 drug products, zero have been discontinued.Product id = 2114
Application Number = 20825
Date of Application = 5,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = GEODON
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 20MG BASE
----
Product id = 2115
Application Number = 20825
Date of Application = 5,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = GEODON
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 2116
Application Number = 20825
Date of Application = 5,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = GEODON
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 2117
Application Number = 20825
Date of Application = 5,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = GEODON
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 14705
Application Number = 21483
Date of Application = 29,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = GEODON
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 10MG BASE/ML
----
Product id = 3829
Application Number = 77560
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3830
Application Number = 77560
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3831
Application Number = 77560
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3832
Application Number = 77560
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 3821
Application Number = 77561
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3822
Application Number = 77561
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3823
Application Number = 77561
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3824
Application Number = 77561
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 3837
Application Number = 77562
Date of Application = 1,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3838
Application Number = 77562
Date of Application = 1,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3839
Application Number = 77562
Date of Application = 1,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3840
Application Number = 77562
Date of Application = 1,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 3825
Application Number = 77565
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3826
Application Number = 77565
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3827
Application Number = 77565
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3828
Application Number = 77565
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 3841
Application Number = 90348
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3842
Application Number = 90348
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3843
Application Number = 90348
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3844
Application Number = 90348
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 3833
Application Number = 202395
Date of Application = 10,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3834
Application Number = 202395
Date of Application = 10,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 3835
Application Number = 202395
Date of Application = 10,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3836
Application Number = 202395
Date of Application = 10,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ZIPRASIDONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----